- Details
- Written by: Editor
- Hits: 398
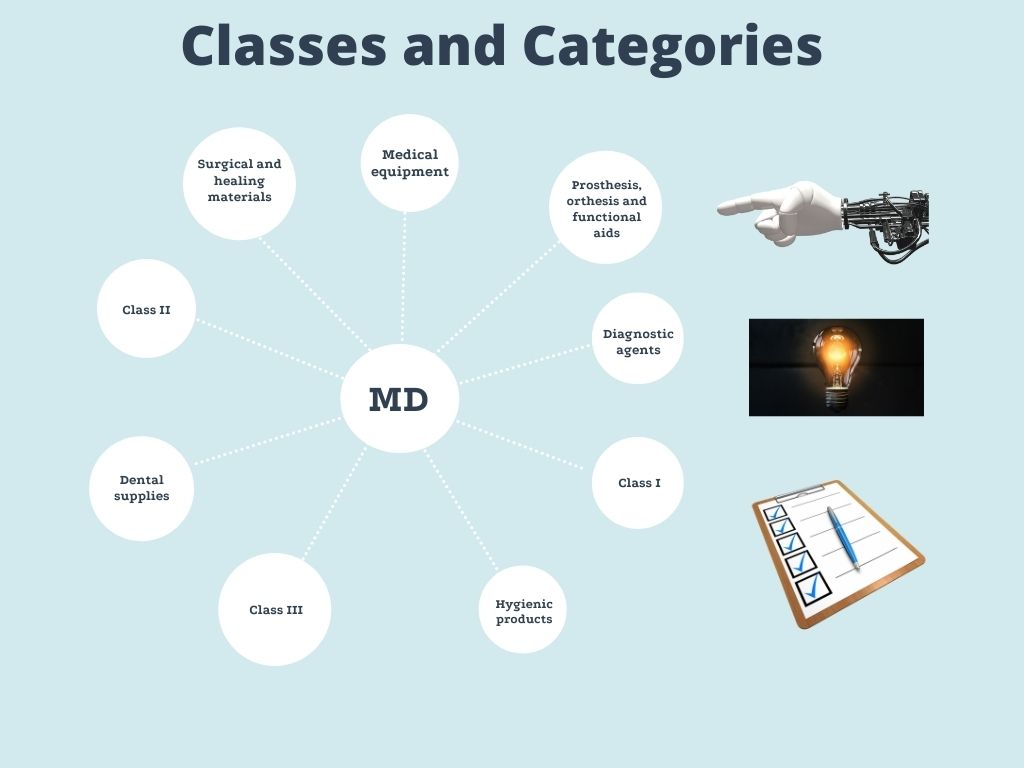
In Mexico, Medical Devices (MD's) are classified by the risk that their use represents and by the category to which they belong.
The definitions of class and category are indicated in the General Act of Health (Ley General de Salud), the Health Care Supplies Regulation (Reglamento de Insumos para la Salud), the Pharmacopoeia of the United Mexican States - Supplement for Medical Devices and the applicable Mexican Official Standards.
The knowledge of the class and category of a MD is very important to obtain the Marketing Authorization Number, since this classification gives us the guideline to know the amount for the payment of rights and the technical documentation that we will have to present to the sanitary authority.
The following are the definitions of the classes and categories described in the aforementioned regulatory framework:
CLASS
Class I: those health care supplies known in medical practice and whose safety and efficacy are proven and, generally, are not introduced into the organism
Class II: those health care supplies known in medical practice and which may have variations in the material with which they are manufactured or in their concentration and, generally, are introduced into the organism for less than thirty days.
Class III: those health care supplies new or recently accepted in medical practice, or that are introduced into the organism and remain in it for more than thirty days.
CATEGORY
I. Medical equipment: apparatus, accessories and instruments for specific use, intended for medical or surgical care or for procedures of exploration, diagnosis, treatment and rehabilitation of patients, as well as those for biomedical research activities.
II. Prosthesis, orthesis and functional aids: those devices intended to substitute or complement a function, an organ or a tissue of the human body.
III. Diagnostic agents: all supplies including antigens, antibodies, calibrators, verifiers, reagents, reagent kits, culture and contrast media and any other similar that may be used as an auxiliary of other clinical or paraclinical procedures.
IV. Dental supplies: all substances or materials used for dental health care.
V. Surgical and healing materials: devices or materials that with or without the addition of antiseptics or germicides are used in the surgical practice or in the treatment of solution of continuity, skin lesions or their annexes.
VI. Hygienic products: materials and substances that are applied on the surface of the skin or body cavities and that have pharmacological or preventive action.
Nowadays, MD´s cover a very wide field, ranging from widely known MD´s such as dressings, gloves, syringes to highly specialized MD´s such as robotic units for surgery, medicated stents, pacemakers, etc. Due to the diversity of MD´s it is important to know and correctly apply the definitions mentioned above, as they help to make the classification process less complex and more accurate.

We hope this information has been of interest to you, let us know if there is a specific regulatory topic you would like us to address.
Don't miss our next blog topic, in which we will be discussing the documentary information for the application of a MD Marketing Authorization Number.
Write comment (0 Comments)- Details
- Written by: Editor
- Hits: 194

New products and technologies are available every day that are fundamental components in the Health Area and it is important to know if these fall within the definition of a Medical Device (MD) described in the Supplement to the Pharmacopoeia of the United Mexican States (FEUM), since its commercialization is regulated in Mexico.
So, how do you know if your product is a medical device?
Below you can find the definition of Medical Device described in the FEUM Supplement, where if the indication for use of your product is within the purposes described in the definition, rest assured that your product is a MD and you will need to register it to the COFEPRIS.
MEDICAL DEVICE: It is any instrument, apparatus, utensil, machine, including the software for its operation, implantable product or material, diagnostic agent, material, substance or similar product, to be employed, alone or in combination, directly or indirectly in human beings; with any of the following purposes of use:
-
- Diagnosis, prevention, surveillance or monitoring, and/or ancillary to the treatment of disease;
- Diagnosis, surveillance or monitoring, treatment, protection, absorption, drainage, or aid in the healing of an injury;
- Substitution, modification, or support of anatomy or a physiological process;
- Life support;
- Conception control;
- Disinfection of medical devices;
- Disinfecting substances;
- Provision of information through an in vitro examination of samples taken from the human body, for diagnostic purposes;
- Devices incorporating tissues of animal and/or human origin;
- Devices used in In Vitro fertilization and assisted reproductive technologies;
And whose primary purpose of use is not through pharmacological, immunological or metabolic mechanisms, however, they can be assisted by these means to achieve their function.

If you got this far and you think your product is indicated for some of the aforementioned purposes, but still have doubts, come to us, at GMVR we support you to analyze whether your product falls within the definition of MD, or if you already have the certainty that your product is a MD, we help you to carry out the Sanitary Registration process.
Don't miss our next blog topic, where we will discuss in detail the categories of medical devices, as well as their classification based on health risk.
Write comment (0 Comments)

