- Details
- Written by: Editor
- Hits: 293
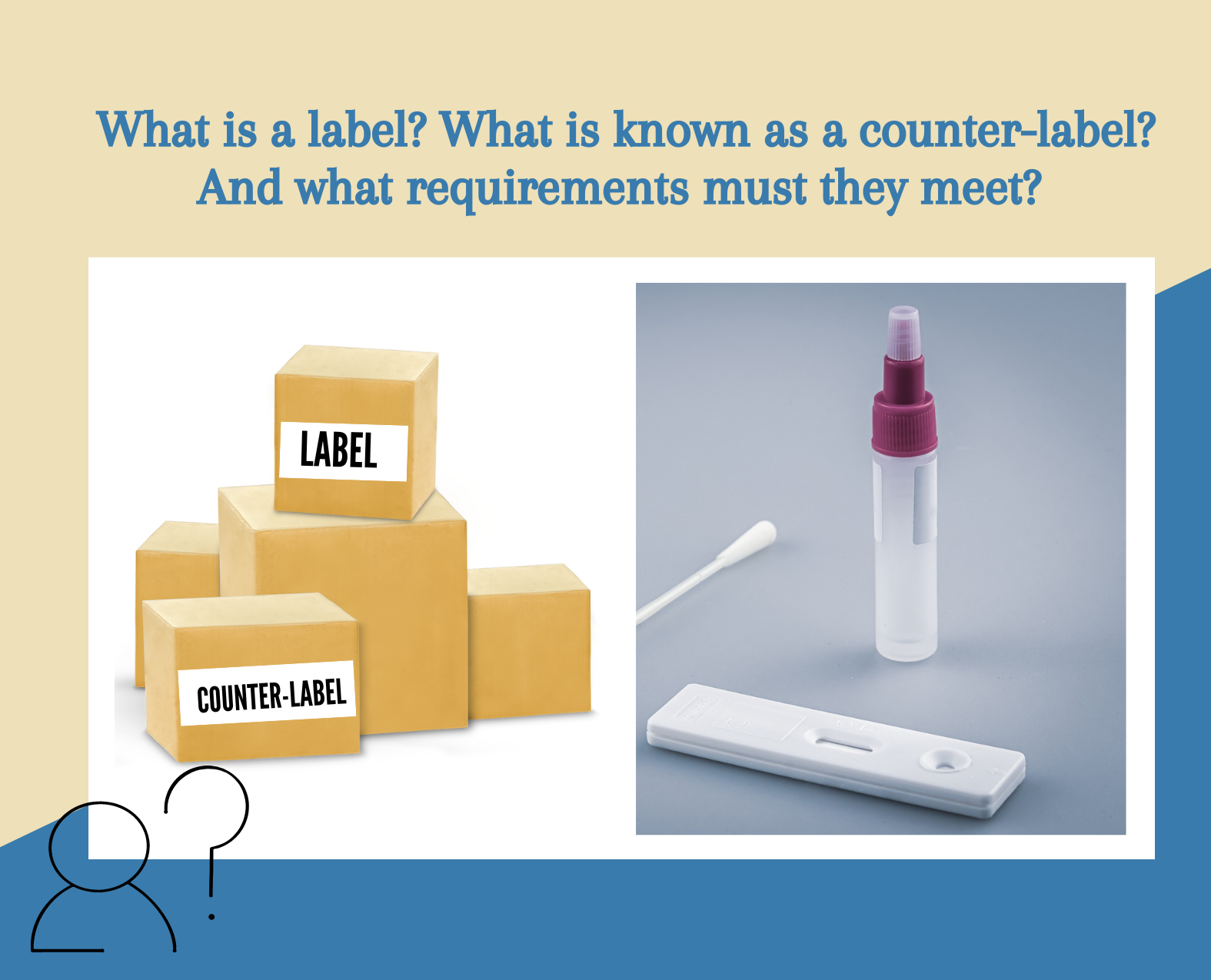
If you have ever wondered what a label is and what is known as a "contraetiqueta" (counter-label), as well as the requirements they must meet, this article from our blog is perfect for you.
In this blog post, we will guide you to understand and reinforce this information and familiarize yourself with the regulatory framework that establishes the characteristics that labels and counter-labels must have.
To begin with, it is important to understand what is indicated in Section II of Article 179 of the Health Supplies Regulation (RIS) and NOM-137-SSA1-2008.
Article 179 of the RIS lists the requirements to obtain the Sanitary Registration of a Medical Device (MD). Number II establishes as a requirement for the project of the label in the Spanish language. Additionally, the same article mentions that the label must comply with the corresponding standard's terms, NOM-137-SSA1-2008.
NOM 137-SSA1 establishes the minimum requirements that must be met to communicate information to users on the labeling of medical devices, whether they are of national or foreign origin, marketed, or intended for use in Mexico. This includes medical equipment; prosthetics, orthesis, and functional aids; diagnostic agents; dental supplies; surgical and healing materials; and hygiene products.
Labels allow for the communication of information to users, and according to the definition of NOM-137-SSA1-2008, they refer to any label, tag, inscription, mark, or graphic image that is written, printed, stenciled, embossed, engraved, adhered, or sealed on any material that can contain the medical device, including the packaging itself.
Unlike the label, the counter-label contains additional or mandatory minimum health and commercial information when the original label does not partially or fully comply with NOM-137-SSA1-2008.
Now that we have covered the regulatory framework and definitions, it's time to review the more practical part of the requirements we must meet to avoid observations from the Health Authority.
The answer is not so simple, as it largely depends on the class, category, and specific characteristics of our MD. However, in general terms, MD labels must comply with the following:
- They must provide complete or additional information (counter-label) in the Spanish language, in accordance with the terms of NOM 137-SSA1. This includes, among others:
- Generic denomination.
- Distinctive denomination.
- Manufacturer's information.
- Country of origin.
- Registration number.
- Lot number or serial number.
- Contents.
- Instructions for use.
- Information about any adverse incidents that may occur due to the use of the product.
- Warning or cautionary statements, if necessary.
- For sterile products, the following statements or symbols must be included:
- "Sterile product."
- "Sterility of the product is not guaranteed if the primary packaging shows signs of prior breakage."
- "Sterilized with ethylene oxide."
- "Sterilized with gamma radiation."
- "Sterilized with dry or moist heat."
- "Processed using aseptic techniques" or other similar statements.
- If applicable, include "Non-toxic," "Pyrogen-free," or other related statements.
- Single-use products must bear the following statements or symbols:
- "Disposable."
- "For single-use only" or similar expressions.
- Reusable sterile medical devices must indicate the methodology for their re-sterilization.
- Symbols for units of measurement must follow the units of the General System of Units of Measurement.
- For formulated devices:
- Include the qualitative formula or the declaration of active ingredients or drug substances contained, as applicable.
- For diagnostic agents, include the statement:
- "Diagnostic agent" and, if applicable, "For exclusive use in Clinical Laboratories or Cabinets."
- The symbols included in normative Appendices A and informative Appendix B of NOM-137-SSA-2008 can be optionally used.
- If applicable, the label or counter-label must include the information indicated in the specific current standards for the product or corresponding pharmacopoeial monographs.
- The label project is submitted in a single copy and in a WORD file and must include the listing of presentations with the description and the code or catalog number of each presentation of the medical device, when necessary.
In conclusion, we can say that both the label and the counter-label play a crucial role in the marketing of MDs. While the original label provides the required basic information, the counter-label acts as a backup, ensuring that no important details are left out. Together, these two pieces form a complete set of necessary data for the sale and proper use of MDs.
We would love to hear your opinion on this topic and if there are any other topics related to Health Regulation that you would like us to address in future articles.
If you have any questions about this topic or need assistance with any procedure before COFEPRIS, do not hesitate to contact us at (+52) 55 6173 2592. We are here to help you with your project.
Write comment (0 Comments)- Details
- Written by: Editor
- Hits: 462
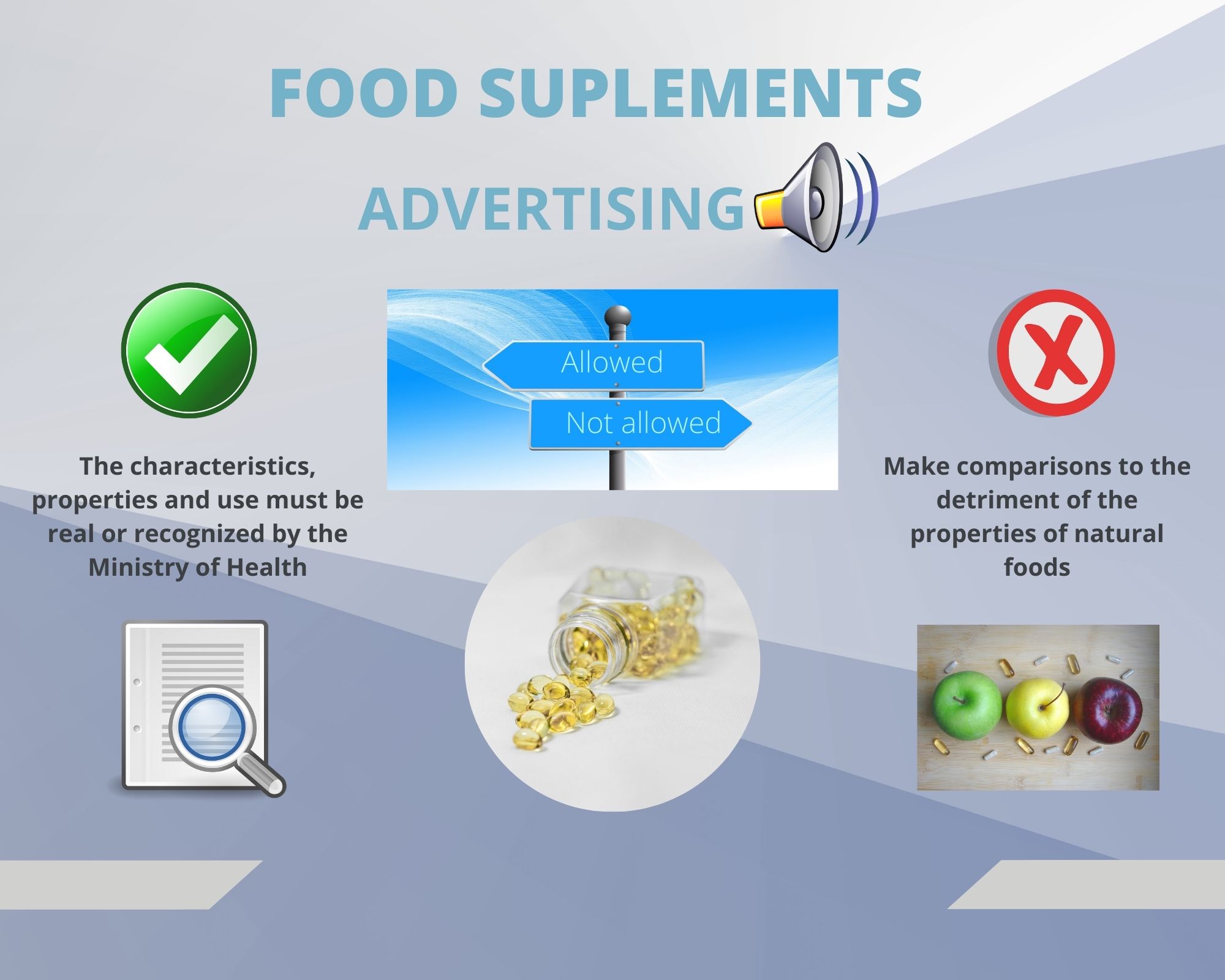
In our blog of May 18, 2022, we have touched on the regulatory framework of Food Supplements, and now it is time to talk about their Advertising.
The advertising of Food Supplements is an extremely important issue, since today many properties are attributed to them that can be misleading and cause damage to the health of the population, for this reason, our Health Authority maintains strictly controlled advertising and asks to have with advertising permission to be able to advertise our food supplements.
To obtain advertising permission, it is necessary for us to know in detail the legends and statements that can be included and what cannot be included in the advertisements.
Below, we leave you in detail what is indicated in the Regulation of the General Health Law in Matters of Advertising where a guide is given to prepare our advertising in such a way that it is guiding and educational regarding the Food Supplement in question.
- It must be consistent with its authorized characteristics or specifications.
- The characteristics, properties and jobs must be real or recognized by the Secretary
- They must be in Spanish, in clear and easily understandable terms for the target audience.
- Provide health information on consumption, which must correspond, where appropriate, to the purposes indicated in the respective authorization.
- Point out the necessary precautions when consumption may cause risk or harm to people's health.
- Contain information on the specifications for proper use, as well as the damage that could be caused to health and incorporate a graphic image of the product to avoid consumer error.
- Be printed in contrasting colors and in the sizes indicated in the Regulations.
- Be written in positive literary forms when it comes to giving instructions for consumption.
- Be written in negative literary forms when it comes to warning the consumer about the risks it may represent.
On the other hand, the advertising of Food Supplements is not allowed to indicate the following, since they may represent a risk to the health of the population:
- Distort or contravene the provisions on nutritional, hygienic and health education established by the Secretary of Health.
- Indicate or lead to believe explicitly or implicitly that the product has the ingredients or properties that it lacks.
- Establish comparisons between products whose ingredients are different, when this may generate risks or damage to health.
- Present as stimulants or modifiers of the physical or mental state of people, except those cases that have been recognized by the Secretariat of Health.
- Induce or promote eating habits that are harmful to health.
- Affirm that the product fulfills by itself the nutritional requirements of the human being.
- Attribute to industrialized foods a higher or different nutritional value than they have.
- Make comparisons to the detriment of the properties of natural foods.
- Express or suggest, through real or fictional characters, that the ingestion of these products provides people with extraordinary characteristics or abilities.
- Declare properties that cannot be verified, or that the products are useful to prevent, alleviate, treat or cure a disease, disorder or physiological state.
- Do not associate with alcoholic beverages and tobacco.
- Those that are held as means to reduce the consumption of nutrients may not be mentioned as dietary and precautionary messages will be established regarding the possible effects that their consumption could cause.
- Names, figures and statements related to diseases, symptoms, syndromes, anatomical data, physiological phenomena should not be used.
Finally, when food supplements are advertised, the legend that the Ministry of Health determines in the advertising authorization based on the health risks that the product represents must be included.
As we could see, the Sanitary Control of the Advertising of Food Supplements is well defined and it is our duty to comply with it to avoid risks to the health of the population that depends a lot on what is sent to them through advertisements.
If you have any questions about this topic or about requesting any procedure before COFEPRIS, contact us at number 55 6167 7092, we can help you with your project.
Give us your opinion on this topic addressed in our blog and share with us if you have any topic in Health Regulation that you want us to review in the next issue.
Write comment (0 Comments)- Details
- Written by: Editor
- Hits: 416

In our past broadcasts, we have addressed the different types of medicines and about generic medicines and new molecules, and now it is time to deal with the Manufacturing License, one of the requirements to be the holder of the Sanitary Registry of a Medicine in Mexico.
In the first place, we have its legal basis, which is found in the Regulation of Health Supplies (Reglamento de Insumos para la Salud), which indicates in its article 168, “to be the holder of the sanitary registration of a medicine, it is required to have a Manufacturing License for a medicine factory or laboratory or biological products for human use. In the case of foreign manufacturers, it is required to have a license, certificate or document that proves that the company has permission to manufacture medicines, issued by the competent authority of the country of origin.
However, what is a Manufacturing License? To answer this question, we can rely on the General Health Law (Ley General de Salud), which in its article 368 indicates that the Manufacturing License is a Sanitary Authorization, which is an administrative act through which the competent Sanitary Authority allows a public or private person, carrying out activities related to human health.
With the above, we can realize that the Manufacturing License must be issued by a competent Sanitary Authority, therefore, if the applicant to be Holder of the Sanitary Registry is a National Manufacturer, the Manufacturing License must be requested to COFEPRIS, below we leave you the link where you can find the format, the instructions and the payment of associated rights for the request for the issuance of a Manufacturing License for the establishment of health supplies (Factory or laboratory of medicines or biological products, for human use).
https://www.gob.mx/cms/uploads/attachment/file/652304/COFEPRIS-05-001-B.pdf
On the other hand, if the applicant to be the Sanitary Registry Holder is a Foreign Manufacturer, the Manufacturing License must be issued by the competent Sanitary Authority of their country of origin.
Now, from the point of view of the application for the sanitary registration of a medicine, we must ask ourselves the following question: what are the characteristics that the Manufacturing License must have to be accepted by our Sanitary Authority when applying for the Sanitary Registry of a Medicine?
If it is of National Manufacture, it is required to present:
- Simple copy of the Manufacturing License and must:
- Indicate the company name and address of the applicant for the health registration (Holder)
- Endorse the Manufacturing Line of the requested medication
If it is of Foreign Manufacture, it is required to present:
- Original or certified copy of the license, certificate or document that proves that the company has permission to manufacture drugs and must:
- Indicate the Company name and address of the Holder.
- Be issued by the Sanitary Authority of the country of origin of the manufacturer who intends to be the holder of the Sanitary Registry
- Endorse the manufacturing line of the requested drug
- Be apostilled or legalized in the country of origin
- Have a translation into Spanish by an expert translator if the source language is different from English.
It is worth mentioning that, in the case of national manufacture, the Manufacturing License of the conditioner, distributor and/or maquiladora must also be presented, if they are involved in the Medication process and the Manufacturing License must endorse the line of the medication process in which they intervene.
Finally, if the medicine belongs to a controlled medicine, independently regardless of whether the Holder is national or foreign, we must also present the Manufacturing License of the Importer and/or Distributor and/or Legal Representative with scope in Warehouse for the Deposit and Distribution of Controlled Medicines or Biological Products, for Human Use.
If you are in the process of assembling of a Dossier to request a Sanitary Registration of a Medication or if you have any questions about Manufacturing Licenses, contact us at 55 6167 7092, we can help you with your project.
Give us your opinion on this topic addressed in our blog and share with us if you have any topic in Health Regulation that you want us to review in the next issue.
Write comment (0 Comments)

